Enivtech will be attending the 2019 International Conference on Thermal Treatment Technologies and Hazardous Waste Combustors (IT3/HWC) October 2-3 in Houston, TX. A paper will be given on “Technology Solutions for Sulfuric Acid Formation and Removal in Liquid Waste and Waste Gas Thermal Oxidizers”. The paper is available for download by clicking the button at the bottom of this blog piece.
Hazardous Waste Combustors (IT3/HWC) October 2-3 in Houston, TX. A paper will be given on “Technology Solutions for Sulfuric Acid Formation and Removal in Liquid Waste and Waste Gas Thermal Oxidizers”. The paper is available for download by clicking the button at the bottom of this blog piece.
Petrochemical plants, refineries, and waste-oil re-refiners operate liquid waste or waste gas thermal oxidizers. The thermal oxidizers need a wet scrubber to neutralize and remove SO2. Flue gas entering the scrubber contains some sulfur trioxide (SO3) which is converted to sulfuric acid (H2SO4) in the presence of water vapor. Sulfuric acid is a submicron liquid aerosol that can pass through downstream equipment, such as a packed bed absorber or a baghouse. Some facilities are now being regulated for H2SO4.  Over the last decade, Envitech has supplied SO2 scrubbers for thermal oxidizers burning sulfur containing compounds in refining applications. Most of these do not have add-on controls for capturing sulfuric acid mist. More recently, however, Envitech has supplied two systems with candle filters for the capture of sulfuric acid mist. Another known system used a wet electrostatic precipitator for the capture of sulfuric acid mist. A potential fourth system with a large gas flow rate with expected SO3 emissions was evaluated for a wet electrostatic precipitator.
Over the last decade, Envitech has supplied SO2 scrubbers for thermal oxidizers burning sulfur containing compounds in refining applications. Most of these do not have add-on controls for capturing sulfuric acid mist. More recently, however, Envitech has supplied two systems with candle filters for the capture of sulfuric acid mist. Another known system used a wet electrostatic precipitator for the capture of sulfuric acid mist. A potential fourth system with a large gas flow rate with expected SO3 emissions was evaluated for a wet electrostatic precipitator.
A thermal oxidizer converts sulfur containing liquid or gaseous waste in the presence of excess oxygen to sulfur dioxide (SO2). A fraction of SO2 is further converted to SO3. The reaction is:
SO2 + 1/2O2 => SO3
The conversion amount is influenced by many factors including the thermal oxidizer operating temperature, residence time, sulfur concentration, amount of excess air, and the presence of catalytic oxides and metal catalysts in the fuel. Literature suggests that even a well-performing thermal oxidizer still converts 1 to 5% of SO2 into SO3. Given the numerous factors influencing the formation of SO3¬, most designers select a conservative estimate of SO3 conversion, even when actual SO3 emissions have been measured, as variations in operation can generate substantially higher conversion.
Once formed in the thermal oxidizer, SO3 reacts with water in the downstream quencher to form sulfuric acid (H2SO4) by the reaction:
SO3(g) + H2O(l) => H2SO4(l)
At temperatures below 350°F, H2SO4 condenses into submicron liquid droplets which are difficult to remove because of their small size. Aerosol droplets pass through a quencher and packed bed absorber. A separate control device is needed for sulfuric acid removal that is suitable for submicron droplets.
Click on the link below to download the IT3/HWC conference paper to learn about “Technology Solutions for Sulfuric Acid Formation and Removal in Liquid Waste and Waste Gas Thermal Oxidizers”. The paper evaluates and compares candle filters versus wet electrostatic precipitators (WESPs) for H2SO4 removal in these types of applications.




 Envitech developed a
Envitech developed a  Another example is a process vent scrubber for a blending facility in South Carolina that produces crop protection products for agricultural markets. The vent stream is 1,500 cfm and includes HCl and water soluble particulate greater than 3 micron in size. The Envitech lab scrubber was configured to include a low pressure drop Venturi for particulate control combined with a packed bed absorber for HCl control. The system includes instruments, control system, recirculation pump, pre-assembled piping, valves, and fittings, interconnect duct, ID fan, and stack.
Another example is a process vent scrubber for a blending facility in South Carolina that produces crop protection products for agricultural markets. The vent stream is 1,500 cfm and includes HCl and water soluble particulate greater than 3 micron in size. The Envitech lab scrubber was configured to include a low pressure drop Venturi for particulate control combined with a packed bed absorber for HCl control. The system includes instruments, control system, recirculation pump, pre-assembled piping, valves, and fittings, interconnect duct, ID fan, and stack.  A different use for a lab scrubber includes an ethylenediamine (
A different use for a lab scrubber includes an ethylenediamine (
 Banbury mixers are used, for instance, to compound rubber material for manufacturing automobile tires. Uncontrolled fumes from the mixers can create a nuisance by settling around the facility. Envitech’s Venturi collision scrubber has been used to control these fumes. The figure on the right shows a typical Venturi collision scrubber for a 25,000 cfm mixer exhaust. The scrubber separates the exhaust into two streams internal to the scrubber. The streams are then directed to two opposing Venturi throats. Recirculated water injected into each throat is atomized into fine droplets as the gas is accelerated. Fume particles and droplets collide and are captured by the atomized water as the steams are recombined into a third Venturi throat. A diffusion section redistributes the gas to a horizontal chevron style mist eliminator to remove entrained water droplets. Water is collected and drained into a common sump and recirculated back to the Venturi throats. A blowdown stream purges the collected material.
Banbury mixers are used, for instance, to compound rubber material for manufacturing automobile tires. Uncontrolled fumes from the mixers can create a nuisance by settling around the facility. Envitech’s Venturi collision scrubber has been used to control these fumes. The figure on the right shows a typical Venturi collision scrubber for a 25,000 cfm mixer exhaust. The scrubber separates the exhaust into two streams internal to the scrubber. The streams are then directed to two opposing Venturi throats. Recirculated water injected into each throat is atomized into fine droplets as the gas is accelerated. Fume particles and droplets collide and are captured by the atomized water as the steams are recombined into a third Venturi throat. A diffusion section redistributes the gas to a horizontal chevron style mist eliminator to remove entrained water droplets. Water is collected and drained into a common sump and recirculated back to the Venturi throats. A blowdown stream purges the collected material.
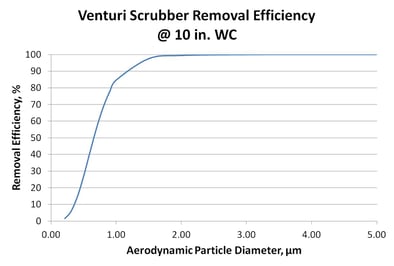 Venturi. It indicates that nearly all particles > 2 micron in size are removed by the Venturi. Performance drops off dramatically, however, for smaller particles. Mechanisms for particulate removal by a Venturi scrubber are discussed in more detail in an earlier
Venturi. It indicates that nearly all particles > 2 micron in size are removed by the Venturi. Performance drops off dramatically, however, for smaller particles. Mechanisms for particulate removal by a Venturi scrubber are discussed in more detail in an earlier  pressure drop over a minimum and maximum gas flow rate. The damper position is governed by proportional-integral-derivative control based on the differential pressure across the throat.
pressure drop over a minimum and maximum gas flow rate. The damper position is governed by proportional-integral-derivative control based on the differential pressure across the throat. 

 In 2009, the US EPA revised the emission limits for the Hospital, Medical, and Infectious Waste Incinerator (
In 2009, the US EPA revised the emission limits for the Hospital, Medical, and Infectious Waste Incinerator (

 A Venturi scrubber is a common air pollution control device that is used to remove particulate. Because it is a wet scrubber, collected particulate is purged in a liquid discharge stream called the blowdown.
A Venturi scrubber is a common air pollution control device that is used to remove particulate. Because it is a wet scrubber, collected particulate is purged in a liquid discharge stream called the blowdown.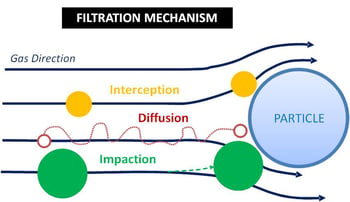
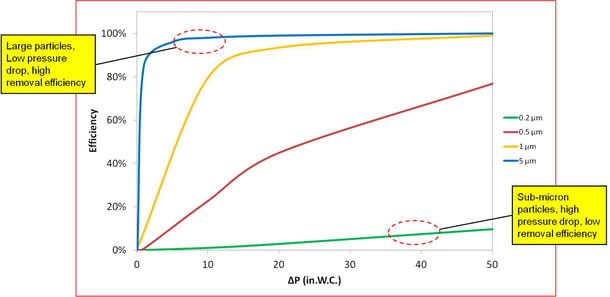

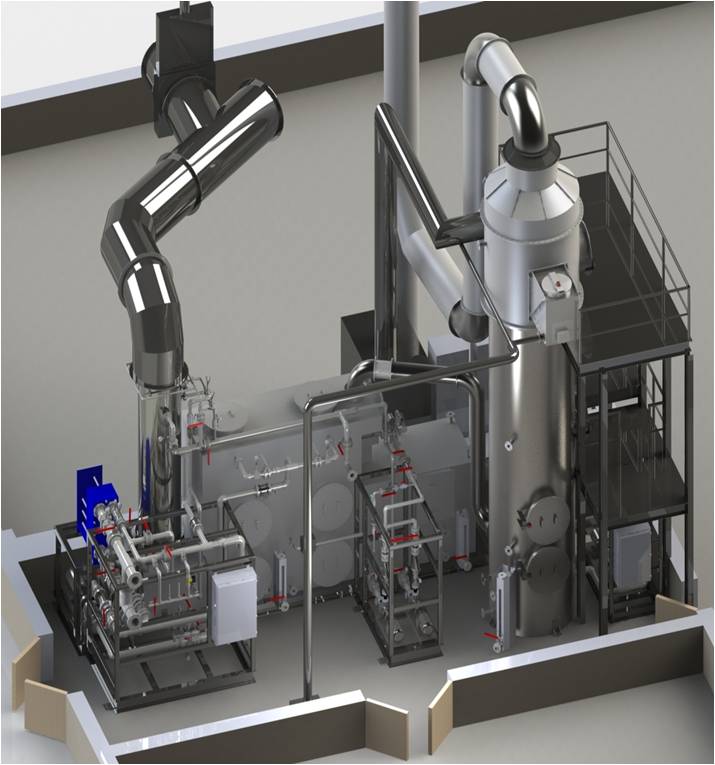 ogram
ogram

 standard. The compliance dates for these rules are fast approaching. Facilities with existing equipment must demonstrate compliance to the new standards by October 2014. Envitech is already under contract with several facilities to retro-fit existing medical waste incinerator scrubbers with add-on control equipment to meet the new standards.
standard. The compliance dates for these rules are fast approaching. Facilities with existing equipment must demonstrate compliance to the new standards by October 2014. Envitech is already under contract with several facilities to retro-fit existing medical waste incinerator scrubbers with add-on control equipment to meet the new standards.  Stack emissions must meet substantially lower limits for Cd, Pb, and Hg. In many cases, this requires add-on controls capable of greater than 90% removal of sub-micron condensed metals. Most facilities are putting on a re-heat and filter package to remove the condensed metals. A few will use wet electrostatic precipitators (WESP) which are more expensive. The ability to meet the new rules using a re-heat and filter package has been demonstrated for lead and cadmium on a commercial and industrial waste incinerator (CISWI). The WESP capability has been demonstrated for reduction of lead emission achieved at a
Stack emissions must meet substantially lower limits for Cd, Pb, and Hg. In many cases, this requires add-on controls capable of greater than 90% removal of sub-micron condensed metals. Most facilities are putting on a re-heat and filter package to remove the condensed metals. A few will use wet electrostatic precipitators (WESP) which are more expensive. The ability to meet the new rules using a re-heat and filter package has been demonstrated for lead and cadmium on a commercial and industrial waste incinerator (CISWI). The WESP capability has been demonstrated for reduction of lead emission achieved at a