Enivtech will be attending the 2019 International Conference on Thermal Treatment Technologies and Hazardous Waste Combustors (IT3/HWC) October 2-3 in Houston, TX. A paper will be given on “Technology Solutions for Sulfuric Acid Formation and Removal in Liquid Waste and Waste Gas Thermal Oxidizers”. The paper is available for download by clicking the button at the bottom of this blog piece.
Hazardous Waste Combustors (IT3/HWC) October 2-3 in Houston, TX. A paper will be given on “Technology Solutions for Sulfuric Acid Formation and Removal in Liquid Waste and Waste Gas Thermal Oxidizers”. The paper is available for download by clicking the button at the bottom of this blog piece.
Petrochemical plants, refineries, and waste-oil re-refiners operate liquid waste or waste gas thermal oxidizers. The thermal oxidizers need a wet scrubber to neutralize and remove SO2. Flue gas entering the scrubber contains some sulfur trioxide (SO3) which is converted to sulfuric acid (H2SO4) in the presence of water vapor. Sulfuric acid is a submicron liquid aerosol that can pass through downstream equipment, such as a packed bed absorber or a baghouse. Some facilities are now being regulated for H2SO4.  Over the last decade, Envitech has supplied SO2 scrubbers for thermal oxidizers burning sulfur containing compounds in refining applications. Most of these do not have add-on controls for capturing sulfuric acid mist. More recently, however, Envitech has supplied two systems with candle filters for the capture of sulfuric acid mist. Another known system used a wet electrostatic precipitator for the capture of sulfuric acid mist. A potential fourth system with a large gas flow rate with expected SO3 emissions was evaluated for a wet electrostatic precipitator.
Over the last decade, Envitech has supplied SO2 scrubbers for thermal oxidizers burning sulfur containing compounds in refining applications. Most of these do not have add-on controls for capturing sulfuric acid mist. More recently, however, Envitech has supplied two systems with candle filters for the capture of sulfuric acid mist. Another known system used a wet electrostatic precipitator for the capture of sulfuric acid mist. A potential fourth system with a large gas flow rate with expected SO3 emissions was evaluated for a wet electrostatic precipitator.
A thermal oxidizer converts sulfur containing liquid or gaseous waste in the presence of excess oxygen to sulfur dioxide (SO2). A fraction of SO2 is further converted to SO3. The reaction is:
SO2 + 1/2O2 => SO3
The conversion amount is influenced by many factors including the thermal oxidizer operating temperature, residence time, sulfur concentration, amount of excess air, and the presence of catalytic oxides and metal catalysts in the fuel. Literature suggests that even a well-performing thermal oxidizer still converts 1 to 5% of SO2 into SO3. Given the numerous factors influencing the formation of SO3¬, most designers select a conservative estimate of SO3 conversion, even when actual SO3 emissions have been measured, as variations in operation can generate substantially higher conversion.
Once formed in the thermal oxidizer, SO3 reacts with water in the downstream quencher to form sulfuric acid (H2SO4) by the reaction:
SO3(g) + H2O(l) => H2SO4(l)
At temperatures below 350°F, H2SO4 condenses into submicron liquid droplets which are difficult to remove because of their small size. Aerosol droplets pass through a quencher and packed bed absorber. A separate control device is needed for sulfuric acid removal that is suitable for submicron droplets.
Click on the link below to download the IT3/HWC conference paper to learn about “Technology Solutions for Sulfuric Acid Formation and Removal in Liquid Waste and Waste Gas Thermal Oxidizers”. The paper evaluates and compares candle filters versus wet electrostatic precipitators (WESPs) for H2SO4 removal in these types of applications.


 An example was given for a captive incinerator at the University of Texas Medical Branch (UTMB) in Galveston, TX. It’s one of the only systems in the United States permitted as a “new” medical waste incinerator according to the EPA HMIWI (hospital, medical, and infectious waste incinerator) MACT standard. This standard has the most challenging emission limits found in industry today. That is because in 2009 the EPA completed a source review and revised the standard based on a MACT-on-MACT analysis. Data used to set limits for each pollutant was individually based on waste feed and not incinerator/scrubber technology performance. This resulted in emission limit reductions for lead (Pb), cadmium (Cd), and dioxins/furans (D/F) that were orders of magnitude below the previous standard and below the capability of installed equipment. The impact of the new standard is discussed in greater detail in a
An example was given for a captive incinerator at the University of Texas Medical Branch (UTMB) in Galveston, TX. It’s one of the only systems in the United States permitted as a “new” medical waste incinerator according to the EPA HMIWI (hospital, medical, and infectious waste incinerator) MACT standard. This standard has the most challenging emission limits found in industry today. That is because in 2009 the EPA completed a source review and revised the standard based on a MACT-on-MACT analysis. Data used to set limits for each pollutant was individually based on waste feed and not incinerator/scrubber technology performance. This resulted in emission limit reductions for lead (Pb), cadmium (Cd), and dioxins/furans (D/F) that were orders of magnitude below the previous standard and below the capability of installed equipment. The impact of the new standard is discussed in greater detail in a  Existing incinerators needed to be upgraded with add-on controls to meet the new standard. New incinerators need air pollution control equipment capable of extraordinarily high removal efficiency for particulate, Pb, Cd, and D/F. A new medium sized incinerator between 200 to 500 lb/hr capacity, has the additional challenge of meeting NOx. A medical waste incinerator can be tuned to a NOx limit of about 130 ppmv. The MACT standard limit for a new medium sized medical waste incinerator was set at 67 ppmv which means NOx abatement is required to guarantee compliance.
Existing incinerators needed to be upgraded with add-on controls to meet the new standard. New incinerators need air pollution control equipment capable of extraordinarily high removal efficiency for particulate, Pb, Cd, and D/F. A new medium sized incinerator between 200 to 500 lb/hr capacity, has the additional challenge of meeting NOx. A medical waste incinerator can be tuned to a NOx limit of about 130 ppmv. The MACT standard limit for a new medium sized medical waste incinerator was set at 67 ppmv which means NOx abatement is required to guarantee compliance.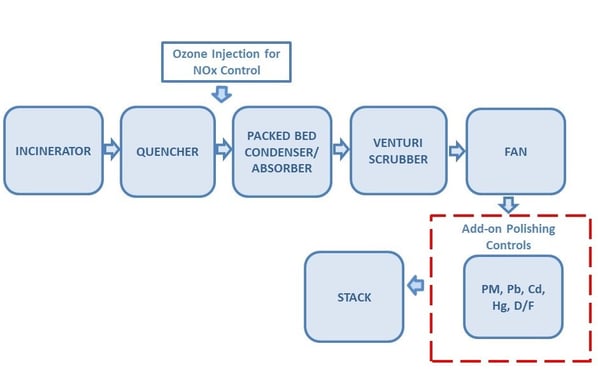
 e of the condenser/absorber is increased to provide sufficient residence time for ozone-NOx reactions to occur. Ozone is highly selective for NOx relative to other combustion products. The NOx is rapidly converted to water soluble species. NO and O3 react to form NO2 and O2. NO2 and O2 react to form N2O5 and O2. N2O5 and water react to form 2HNO3 which is readily absorbed with caustic solution.
e of the condenser/absorber is increased to provide sufficient residence time for ozone-NOx reactions to occur. Ozone is highly selective for NOx relative to other combustion products. The NOx is rapidly converted to water soluble species. NO and O3 react to form NO2 and O2. NO2 and O2 react to form N2O5 and O2. N2O5 and water react to form 2HNO3 which is readily absorbed with caustic solution.
 previously blogged about include refinery sulfur recovery unit tail gas treatment units (
previously blogged about include refinery sulfur recovery unit tail gas treatment units (
 Envitech developed a
Envitech developed a  Another example is a process vent scrubber for a blending facility in South Carolina that produces crop protection products for agricultural markets. The vent stream is 1,500 cfm and includes HCl and water soluble particulate greater than 3 micron in size. The Envitech lab scrubber was configured to include a low pressure drop Venturi for particulate control combined with a packed bed absorber for HCl control. The system includes instruments, control system, recirculation pump, pre-assembled piping, valves, and fittings, interconnect duct, ID fan, and stack.
Another example is a process vent scrubber for a blending facility in South Carolina that produces crop protection products for agricultural markets. The vent stream is 1,500 cfm and includes HCl and water soluble particulate greater than 3 micron in size. The Envitech lab scrubber was configured to include a low pressure drop Venturi for particulate control combined with a packed bed absorber for HCl control. The system includes instruments, control system, recirculation pump, pre-assembled piping, valves, and fittings, interconnect duct, ID fan, and stack.  A different use for a lab scrubber includes an ethylenediamine (
A different use for a lab scrubber includes an ethylenediamine (
 A common application for small scrubber systems is an emergency vent scrubber for l
A common application for small scrubber systems is an emergency vent scrubber for l
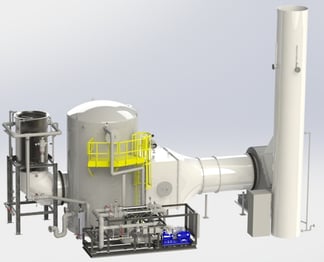

 incinerator. The incinerator exhaust is ducted to a metal quencher (shown in the foreground). The hot gas enters the top of the quencher and flows vertically downward. The gas then elbows into the bottom of a vertical packed bed scrubber (shown in the background). The gas passes upward through the packed bed as re-circulated water flows downward, counter-current to the gas from the top of the packed bed. Water from the quencher and packed bed is collected in the sump and re-circulated back to the quencher and packed bed. An entrainment separator at the top of the scrubber removes entrained water droplets. After exiting the scrubber vessel, an interconnect duct transports the gas to a induced draft fan located at grade.
incinerator. The incinerator exhaust is ducted to a metal quencher (shown in the foreground). The hot gas enters the top of the quencher and flows vertically downward. The gas then elbows into the bottom of a vertical packed bed scrubber (shown in the background). The gas passes upward through the packed bed as re-circulated water flows downward, counter-current to the gas from the top of the packed bed. Water from the quencher and packed bed is collected in the sump and re-circulated back to the quencher and packed bed. An entrainment separator at the top of the scrubber removes entrained water droplets. After exiting the scrubber vessel, an interconnect duct transports the gas to a induced draft fan located at grade.


 cinerator and waste heat boiler treats the TGTU off-gas before it is exhausted to atmosphere. During normal operations, there is very little SO2 emissions due to the high sulfur recovery. However, TGTU upsets can occur several times per year which sends unrecovered sulfur to the incinerator. During these upsets, SO2 emissions can be as high as 1 tph or more for a period of 8 to 12 hours.
cinerator and waste heat boiler treats the TGTU off-gas before it is exhausted to atmosphere. During normal operations, there is very little SO2 emissions due to the high sulfur recovery. However, TGTU upsets can occur several times per year which sends unrecovered sulfur to the incinerator. During these upsets, SO2 emissions can be as high as 1 tph or more for a period of 8 to 12 hours.
 from the earth’s geothermal resources into electrical energy. The fluids are recovered in the
from the earth’s geothermal resources into electrical energy. The fluids are recovered in the


